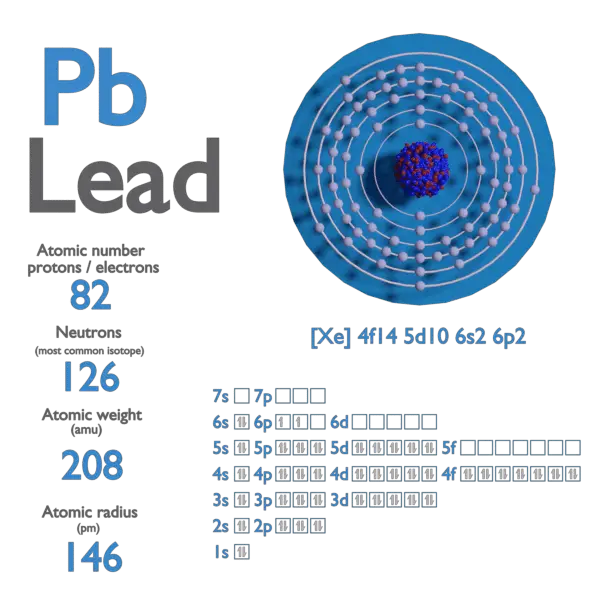
Lead 210 Protons Neutrons Electrons . Lead has 82 protons and electrons in its structure. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. In other words, it's the sum of the number of. the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. The total number of neutrons in the nucleus of an atom is called the neutron number. In nature, the chemical element. describe the locations, charges, and masses of the three main subatomic particles. (the more massive 209 bi, long considered to be. 133 rows lead is the element with the heaviest stable isotope, 208 pb. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The sum of the number of protons and neutrons of an atomic nucleus.
from www.nuclear-power.com
Lead has 82 protons and electrons in its structure. the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. In nature, the chemical element. In other words, it's the sum of the number of. The sum of the number of protons and neutrons of an atomic nucleus. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. (the more massive 209 bi, long considered to be. 133 rows lead is the element with the heaviest stable isotope, 208 pb. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. describe the locations, charges, and masses of the three main subatomic particles.
Lead Atomic Number Atomic Mass Density of Lead
Lead 210 Protons Neutrons Electrons The total number of neutrons in the nucleus of an atom is called the neutron number. Lead has 82 protons and electrons in its structure. The sum of the number of protons and neutrons of an atomic nucleus. the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. In other words, it's the sum of the number of. describe the locations, charges, and masses of the three main subatomic particles. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. In nature, the chemical element. The total number of neutrons in the nucleus of an atom is called the neutron number. (the more massive 209 bi, long considered to be. 133 rows lead is the element with the heaviest stable isotope, 208 pb.
From animalia-life.club
Electron Proton Neutron Lead 210 Protons Neutrons Electrons the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. In other words, it's the sum of the number of. describe the locations, charges, and masses of the three main subatomic particles. The sum of the number of protons and neutrons of an atomic nucleus. Lead has 82 protons and electrons in. Lead 210 Protons Neutrons Electrons.
From www.numerade.com
SOLVED Question 5 12 pts 1. Fill in the missing information (12 pts Lead 210 Protons Neutrons Electrons In nature, the chemical element. (the more massive 209 bi, long considered to be. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of. 133 rows lead is the element with the heaviest stable isotope, 208 pb. protons, neutrons and electrons of all elements are. Lead 210 Protons Neutrons Electrons.
From exoxpojqw.blob.core.windows.net
Lead 214 Protons Neutrons Electrons at Heather Doss blog Lead 210 Protons Neutrons Electrons In nature, the chemical element. describe the locations, charges, and masses of the three main subatomic particles. 133 rows lead is the element with the heaviest stable isotope, 208 pb. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. the difference between the individual lead isotopes lies in the. Lead 210 Protons Neutrons Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead 210 Protons Neutrons Electrons describe the locations, charges, and masses of the three main subatomic particles. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. 133 rows lead is the element with the heaviest stable isotope, 208 pb. (the more massive 209 bi, long considered to be. the difference. Lead 210 Protons Neutrons Electrons.
From www.youtube.com
Atomic Structure Protons, Electrons & Neutrons YouTube Lead 210 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. 133 rows lead is the element with the heaviest stable isotope, 208 pb. The sum of the number of protons and neutrons of an atomic nucleus. Lead has 82 protons and electrons in its structure. The total number of neutrons in the. Lead 210 Protons Neutrons Electrons.
From lessondbmarshall.z4.web.core.windows.net
Protons Neutrons And Electrons Chart Lead 210 Protons Neutrons Electrons Lead has 82 protons and electrons in its structure. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. In nature, the chemical element. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The sum of the number of protons and. Lead 210 Protons Neutrons Electrons.
From guidelibperplexing.z13.web.core.windows.net
Diagram Of Protons Neutrons And Electrons Lead 210 Protons Neutrons Electrons In other words, it's the sum of the number of. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. In nature, the chemical element. the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. Lead has 82 protons and electrons in. Lead 210 Protons Neutrons Electrons.
From learningschoolamangisico.z14.web.core.windows.net
Protons Neutrons And Electrons In An Atom Lead 210 Protons Neutrons Electrons describe the locations, charges, and masses of the three main subatomic particles. In other words, it's the sum of the number of. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. 133 rows lead is the element with the heaviest stable isotope, 208 pb. The total. Lead 210 Protons Neutrons Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead 210 Protons Neutrons Electrons the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. 133 rows lead is the element with the heaviest stable isotope, 208 pb. Lead has 82 protons and electrons in its structure. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list +. Lead 210 Protons Neutrons Electrons.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead 210 Protons Neutrons Electrons In other words, it's the sum of the number of. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. 133 rows lead is the element with the heaviest stable isotope, 208 pb. In nature, the chemical element. (the more massive 209 bi, long considered to be. describe the locations, charges,. Lead 210 Protons Neutrons Electrons.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead 210 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. In other words, it's the sum of the number of. (the more massive 209 bi, long considered to be. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The total number. Lead 210 Protons Neutrons Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead 210 Protons Neutrons Electrons 133 rows lead is the element with the heaviest stable isotope, 208 pb. The total number of neutrons in the nucleus of an atom is called the neutron number. In nature, the chemical element. In other words, it's the sum of the number of. the difference between the individual lead isotopes lies in the number of neutrons in. Lead 210 Protons Neutrons Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead 210 Protons Neutrons Electrons the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. The sum of the number of protons and neutrons of an atomic nucleus. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. describe the locations, charges, and masses of the. Lead 210 Protons Neutrons Electrons.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead 210 Protons Neutrons Electrons describe the locations, charges, and masses of the three main subatomic particles. In nature, the chemical element. The following table shows the atomic nuclei that are isotonic (same neutron number n = 128) and. (the more massive 209 bi, long considered to be. The total number of neutrons in the nucleus of an atom is called the neutron number.. Lead 210 Protons Neutrons Electrons.
From slideplayer.com
Chapter 4 Review “Atomic Structure” ppt download Lead 210 Protons Neutrons Electrons Lead has 82 protons and electrons in its structure. protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. The sum of the number of protons and neutrons of an atomic nucleus.. Lead 210 Protons Neutrons Electrons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Tin? Lead 210 Protons Neutrons Electrons 133 rows lead is the element with the heaviest stable isotope, 208 pb. The total number of neutrons in the nucleus of an atom is called the neutron number. describe the locations, charges, and masses of the three main subatomic particles. In nature, the chemical element. (the more massive 209 bi, long considered to be. protons, neutrons. Lead 210 Protons Neutrons Electrons.
From dxoadjnkf.blob.core.windows.net
Bromine Protons Electrons And Neutrons at Stephanie Porter blog Lead 210 Protons Neutrons Electrons 133 rows lead is the element with the heaviest stable isotope, 208 pb. The sum of the number of protons and neutrons of an atomic nucleus. the difference between the individual lead isotopes lies in the number of neutrons in the nucleus. Lead has 82 protons and electrons in its structure. The total number of neutrons in the. Lead 210 Protons Neutrons Electrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C013/1639 Science Photo Library Lead 210 Protons Neutrons Electrons The total number of neutrons in the nucleus of an atom is called the neutron number. 133 rows lead is the element with the heaviest stable isotope, 208 pb. (the more massive 209 bi, long considered to be. describe the locations, charges, and masses of the three main subatomic particles. In nature, the chemical element. protons, neutrons. Lead 210 Protons Neutrons Electrons.